Abstract
Background: Isocitrate dehydrogenase 1 mutations (IDH1m) occur in 7-14% of AML patients and 3% of MDS patients. Mutated IDH1 metabolizes alpha-ketoglutarate into 2-hydroxyglutarate (2-HG), an oncometabolite that impairs differentiation. FT-2102 is an oral, highly potent, selective small molecule inhibitor of mutated IDH1, with the therapeutic potential to restore normal cellular differentiation. Azacitidine (AZA), a hypomethylating agent (HMA), is a standard of care for MDS and for AML patients unfit for intensive therapy. We present Phase 1 clinical data from the ongoing Phase 1/2 study of FT-2102 in combination with azacitidine (FT-2102+AZA) in patients with IDH1m AML and MDS (CT.gov: NCT02719574).
Methods: A Phase 1 study was initiated to evaluate the safety, PK/PD, and antileukemic activity of FT-2102 in patients with IDH1m AML or MDS and included both dose escalation and expansion. FT-2102 was administered daily (150 QD or 150 BID) until disease progression or unacceptable toxicity; AZA was administered at 75 mg/m2 IV or SC daily for 7 days of each 28-day cycle. Eligible patients had IDH1m AML/MDS that was relapsed/refractory (R/R) or treatment-naïve (TN), were eligible for AZA therapy and had adequate liver and renal function. Prior IDH1 inhibitors and hypomethylating agents were allowed, and there were no restrictions for concomitant non-anticancer medications. Investigator-assessed responses were per modified IWG 2003/2006 criteria. Efficacy was assessed at Cycle 2 Day 1 and as clinically indicated thereafter. Adverse events (AEs) were assessed throughout the study per NCI CTCAE version 4.03.
Results: At the time of the data cutoff, 07 April 2018, 27 patients had been enrolled and 26 had been treated with FT-2102+AZA, with a median of 3 months on treatment (range: 0.2 to 12 months). Of the 27 enrolled patients, 24 had AML (20 R/R; 4 TN) and 3 patients had MDS (all TN). The median number of prior antileukemia therapies was 3 (range: 0-5) in the AML patients. Doses of FT-2102 were 150 mg QD (n=7) and 150 mg BID (n=20). Thirteen (50%) patients discontinued treatment, with the most common reasons for treatment discontinuation were hematopoietic stem cell transplant (n=4), death (n=2), and progressive disease (n=2). No patients discontinued due to an AE.
Severe (≥ Grade 3) AEs occurring in ≥ 5% of patients included febrile neutropenia (27%), anemia, thrombocytopenia, leukocytosis (19% each), fatigue, hypertension, neutropenia (15% each), nausea, pneumonia, hypokalemia (12% each) and abdominal pain (8%). Three patients had differentiation syndrome (IDH-DS), which resolved with FT-2102 temporary interruption, and treatment with dexamethasone, hydroxyurea, and supportive care in all three. Five patients died within 28-days of the last dose of study drug, none of which were considered related to FT-2102+AZA. No DLTs were observed during dose escalation.
PK analysis demonstrated no impact of AZA on steady-state exposure of FT-2102. 2-HG levels to normal levels by C2D1.
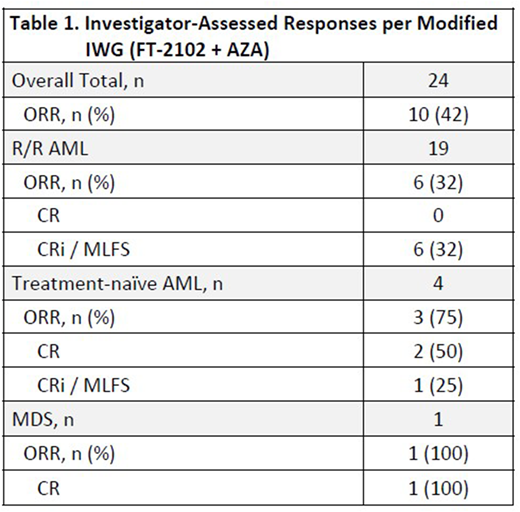
Table 1 shows the Investigator-assessed ORR per IWG. Responses have been seen as early as 1 month on treatment, and stable disease for greater than 6 months; 50% of patients remain on treatment.
Conclusions: FT-2102+AZA is well tolerated in AML and MDS, with 150 mg BID selected as the RP2D based on safety, PK and PD (2-HG) response. Clinical activity has been observed, with an ORR of 42%. Durable CRs (>12 months) have been observed. The current data support the continued evaluation of FT-2102 150 mg BID+AZA in the Phase 2 study. Three Phase 2 combination therapy cohorts are currently open for enrollment in patients < 60 years with R/R AML or MDS naïve to HMA and IDH1m inhibitors; R/R AML or MDS with inadequate response/PD on HMA, and R/R AML or MDS who have progressed on a prior IDH1m inhibitor. Updated clinical data will be available at the time of presentation.
Cortes:Pfizer: Consultancy, Research Funding; Arog: Research Funding; Astellas Pharma: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Novartis: Consultancy, Research Funding. Schiller:Celator/Jazz Pharmaceuticals: Research Funding; Pharmacyclics: Research Funding. Lee:AstraZeneca: Consultancy; Clinipace: Consultancy; Karyopharm Therapeutics Inc: Consultancy; LAM Therapeutics: Research Funding; Amgen: Consultancy. Wang:Amgen: Consultancy; Jazz: Speakers Bureau; Amgen: Consultancy; Jazz: Speakers Bureau; Novartis: Speakers Bureau; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Speakers Bureau; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees. Ferrell:Incyte: Research Funding. Jonas:LP Therapeutics: Research Funding; Genentech/Roche: Research Funding; Incyte: Research Funding; Daiichi Sankyo: Research Funding; Kalobios: Research Funding; Glycomimetics: Research Funding; AbbVie: Consultancy, Research Funding; Forma: Research Funding; Accelerated Medical Diagnostics: Research Funding; Tolero: Consultancy; Esanex: Research Funding; Celgene: Consultancy, Research Funding; Amgen: Consultancy; Pharmacyclics: Research Funding. Wei:Novartis: Honoraria, Other: Advisory committee, Research Funding, Speakers Bureau; Servier: Consultancy, Honoraria, Other: Advisory committee, Research Funding; Pfizer: Honoraria, Other: Advisory committee; Abbvie: Honoraria, Other: Advisory board, Research Funding, Speakers Bureau; Amgen: Honoraria, Other: Advisory committee, Research Funding; Celgene: Honoraria, Other: Advisory committee, Research Funding. Montesinos:Novartis: Research Funding, Speakers Bureau; Daiichi Sankyo: Consultancy, Speakers Bureau. Kelly:Forma Therapeutics Inc.: Employment. Li:Forma Therapeutics Inc.: Employment. Sweeney:Forma Therapeutics Inc.: Employment. Watson:Forma Therapeutics Inc.: Employment. Mohamed:Forma Therapeutics Inc.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal